
Recently on Cyclingnews.com |
Potentially fatal disease linked to artificial EPO
A Cyclingnews special report
A recently published study has revealed that use of artificial erythropoietin (EPO) can lead to the total shutdown of red blood cell production. Patients treated with or misuse EPO are at risk of developing neutralising anti-EPO antibodies, inhibiting the body's ability to produce red blood cells. The potentially fatal condition is known as pure red-cell aplasia, as Anthony Tan discovers.
Many names, two companiesEprex, the most common brand of EPO sold almost exclusively to the European and Canadian markets, is manufactured by subsidiaries of healthcare giant Johnson & Johnson. Eprex is also sold under the sub-brands Erypo, Epopen, Epoxitin and Globuren. Eprex's equivalent in the US, marketed as Procrit and Epogen, is produced by Californian-based biotechnology firm Amgen. According to Dr Jay Gehrig, a US-based M.D., users of the Amgen-produced EPO are at much less risk: "Eprex is not identical to the Procrit and Epogen. There have only been two cases of pure red-cell aplasia occurring in persons taking the Amgen-produced EPO. I would guess that most European athletes [using EPO] take Eprex," he says. |
"I THINK many athletes have often ignored slight risks in favour of major gains," says Dr John Mahony, Renal Physician and Head of Kidney Transplantation Unit at Sydney's Royal North Shore Hospital.
"That's why there's been long-term problems with use of androgenic steroids. [The mentality is:] 'take it when we're young, suffer when we're older'. Red-cell aplasia is less of a risk numerically, but devastating when it occurs," warns Dr Mahony.
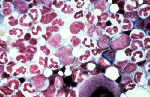 |
Pure Red Cell Aplasia (PRCA) is a condition where a selective reduction in the red blood cell components of the bone marrow results in severe anaemia. The condition differs from aplastic anaemia, where all the bone marrow cells - red, white and platelet cells - are affected. "It seems to be very specific for red cells in relation to the formation of the antibodies," says Dr Mahony.
The study, published by Casadevall et al. in the New England Journal of Medicine[1], documented 13 cases of patients diagnosed with PRCA after using Eprex, a brand of artificial EPO sold mostly to the European and Canadian markets.
In 141 out of a total 150 reported cases of EPO-induced PRCA, Eprex - a Johnson & Johnson brand of erythropoietin - was the brand that led to the condition [see first breakout box]. The onset of antibodies render both the artificial EPO and the person's own endogenous (natural) EPO ineffective - and consequently the bone marrow's normal ability to stimulate production of red blood cells.
Cause of PRCA trigger unknown
“less of a risk numerically, but devastating when
it occurs” -
|
Although there have been more than five companies producing EPO that have been linked to the condition, Johnson & Johnson, not surprisingly, has been the most vigilant in its investigations into possible triggers of pure red-cell aplasia - so far to no avail. To date, no single cause has been identified.
Should legitimate EPO users be afraid?
Robin Parisotto, a high-profile sports scientist from the Sports Haematology and Biochemistry Laboratory at the Australian Institute of Sport (AIS), dismisses the notion.
"Like anything foreign that is introduced to the body, there is always the chance that the body may or will reject it in some way," says Parisotto. "The risk of developing red-cell aplasia is very low, and should be put into perspective when you consider there are some 500,000 patients worldwide currently receiving EPO treatment - where there is a significant increase in quality of life, in addition to prolongation of life."
Dr Jay Gehrig concurs, a US-based M.D and regular Cyclingnews contributor. Having treated several thousand kidney disease patients with "tens of thousands of doses of EPO" over the last 12 years, Dr Gehrig has not seen PRCA occur, and denies any risk for the legitimate user. "In my view, at least here in the USA, EPO is a safe drug when used appropriately," says Dr Gehrig.
Dr Mahony from the Royal North Shore Hospital in Sydney agrees in part with Parisotto and Dr Gehrig: "It's an uncommon condition, but I think the medical community is concerned that the cases have occured, and they've changed their practices to try and prevent further cases from occurring," he says.
Cause of cure also unknown
The inability to identify a source, combined with the minute risk of developing such unique antibodies (less than one in 10,000) means there is no known cure for the condition of pure red-cell aplasia.
Explains Dr Mahony, "There are a various options, because the most appropriate treatment for the disease is unknown. A number of options have been explored, from doing nothing other than [blood] tranfusion and waiting, to steroids - to other immune-suppressing agents that attempt to abolish the antibody. We don't have a fixed protocol because it's such an uncommon condition," says Dr Mahony.
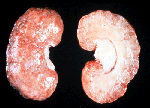 |
The only good news, if it can be called that, is that the condition is rarely fatal in itself. Dr Mahony says that if a patient becomes transfusion dependent, they are unlikely to die solely from being transfusion dependent. Rather, the circumstance and length in which EPO is being used dictates their mortality and what they will die from. For example, if one has renal failure, they may die from events associated with kidney disease (such as a stroke) but will not necessarily die just from being transfusion dependent - although in the long term, it will become more difficult to obtain compatible blood transfusions.
Manufacturing problems linked to PRCA?On July 19 this year, shares of Johnson & Johnson plummeted to a 16-month low after US Federal regulators began probing allegations of fraudulent record keeping at their plant in Puerto Rico. Ortho Biologics, the Puerto Rican subsidiary of Johnson & Johnson, makes one of their best-selling medicines: artificial blood booster Eprex. Recently Eprex has come to the public eye due to its association with the severe anaemic condition known as pure red-cell aplasia. The allegations involve a former employee at the plant, Hector Arce. According to an article published in the New York Times, Arce has filed a lawsuit against J&J, and has alleged he was coerced to falsify data to cover up manufacturing faults. Arce also claims that he was suspended from his job just days before a planned interview with FDA inspectors in 1999. Since Arce's "whistle-blower lawsuit" and its link to the FDA's criminal investigation, J&J has denied there is any link between the investigation and the side effects of Eprex: "Our investigation of the employee's allegations confirm that the integrity of our product has never been compromised, and there is no connection between the allegations and the occurrence of pure red blood-cell aplasia," J&J said in a statement soon after the share collapse on the New York Stock Exchange. Combined sales of both Johnson & Johnson and Amgen produced EPO products exceed US$5 billion worldwide. EPO is by far the best product conceived by genetic engineering and is one of the most used products globally, with more than three million recipients. |
Short-term use of EPO no safety net
The onset of red-cell aplasia also appears to be time-sporadic, and the length of time a patient remains transfusion-dependent is undetermined.
The paper published in the New England Journal of Medicine found severe anaemia developed anywhere from 3 to 67 months during treatment. After discontinuing treatment using erythropoietin, 6 of the 13 patients diagnosed with the condition were able to develop red blood cells on their own after being treated with immunosuppressants. However, three remain transfusion-dependent more than two years later.
"The answer is not known for sure," says Dr Mahony. "Some people have recovered within 12 months; the majority have not done so, and whether they recover or not remains to be seen," he says.
No such thing as "bad" EPO
“There is no such thing as 'bad' EPO” - this
drug was not meant to be used by fit and healthy people" -
|
Robin Parisotto from the Australian Insitute of Sport is adamant that Eprex, or any other brand of EPO for that matter, should not be labelled as a "bad" form of EPO.
"There is no reason to believe that Eprex is 'bad' EPO. This drug was not meant to be used by fit and healthy people, and so in the context of doping in sport, there is no such thing as 'bad' EPO, but rather it should be condemned for its illegal use," says Parisotto.
Risk via subcutaneous adminstration thirty-fold; sales of Aranesp likely to skyrocket
From a sporting context, it is worth noting that the incidence of developing pure red-cell aplasia is associated primarily with the subcutaneous route of administration, as opposed to intravenously. In fact, the propensity to develop PRCA via subcutaneous administration is around thirty times greater.
While the physical risk may be low, from a monetary perspective, the situation is potentially disastrous. Combined global sales of Eprex and Procrit[2] were $3.8 billion in 2001 - and one of Johnson & Johnson's best selling products, equivalent to roughly 4% of total revenues for J&J worldwide.
The entry of Aranesp (also known as NESP), a longer lasting EPO formulation - although more easily detectable - spells good times for Amgen. Furthermore, Amgen's best-selling "standard" EPO brand, Epogen, has had only one reported case of pure red-cell aplasia in 12 years of use.
Conversely, if red-cell aplasia is a rare side effect of all artificial EPO products, it could also have a negative impact on sales of all brands of EPO. However, to date, this theoretical possibility has not been seen in real patients. "There have been so specific cases [of red-cell aplasia] directly related to NESP," Dr Mahony says.
Sporting's future: DynEPO and "genetic" doping
Robin Parisotto says that a new human cell line-derived EPO, DynEPO (pronounced dine e-p-o), will be the next major threat to sporting bodies and drug testing authorities worldwide. DynEPO is now sufficiently similar to naturally-occuring EPO - and in theory, is undectable under current drug testing protocols, including the urine test used by cycling's governing body, the Union Cycliste Internationale (UCI).
Parisotto says the concern is two-fold. "First, DynEPO may not be directly detectable using current testing protocols, so it may well be used indefinitely or continuously by cheating athletes at doses to maintain an allowable haematocrit level. Second, the risk of developing anti-EPO antibodies may also increase as a result. In the future, the spectre of 'genetic' doping will also loom," he says.
How serious do you think the issue of EPO still is within pro cycling? - Tell us your thoughts.
Click here to read the Cyclingnews feature on DynEPO.
References
[1] Nicole Casadevall, Joelle Nataf, Béatrice Viron, Amir Kolta, Jean-Jacques Kiladjian, Philippe Martin-Dupont, Patrick Michaud, Thomas Papo, Valérie Ugo, Irène Teyssandier, B.S., Bruno Varet, and Patrick Mayeux, Ph.D. Pure Red-Cell Aplasia and Antierythropoietin Antibodies in Patients Treated with Recombinant Erythropoietin. N Engl J Med 346: 469-475, February 14, 2002. http://content.nejm.org/cgi/content/abstract/346/7/469; correction: http://content.nejm.org/cgi/content/full/347/6/458
[2] Procrit is developed and manufactured by Amgen, but is resold to cancer patients in the US by Johnson & Johnson. The Amgen brand name is used only for patients undergoing kidney dialysis.
Leon Henderson, Bennett Weintraub, Christopher Martin. Is Pure Red-Cell Aplasia Linked to All Epo Products or Just Eprex? Btech News, February 18, 2002. http://www.bioportfolio.com/news/btech_021802_1.htm
Cazzola M. Further concerns about the medical risks of blood doping. Haematologica 85:561-3, March 2002. http://www.haematologica.ws/2002_03/232.htm
Paul Schik. Pure Red-Cell Aplasia. eMedicine, April 19, 2002. http://www.emedicine.com/med/topic1967.htm
The Initiative. Examining the Immune Responses to Biotech Drugs. The Pew Initiative on Food and Biotechnology, July 30, 2002. http://pewagbiotech.org/newsroom/summaries/display.php3?NewsID=219
Reuters. Johnson & Johnson confirms probe. MSNBC.com, July 19, 2002. http://www.msnbc.com/news/782780.asp#BODY
Lawrence Carrel. Bad PR for Johnson & Johnson. SmartMoney.com, July 19, 2002. http://biz.yahoo.com/smart/020719/20020719one_wond_5.html
